
Director of Research
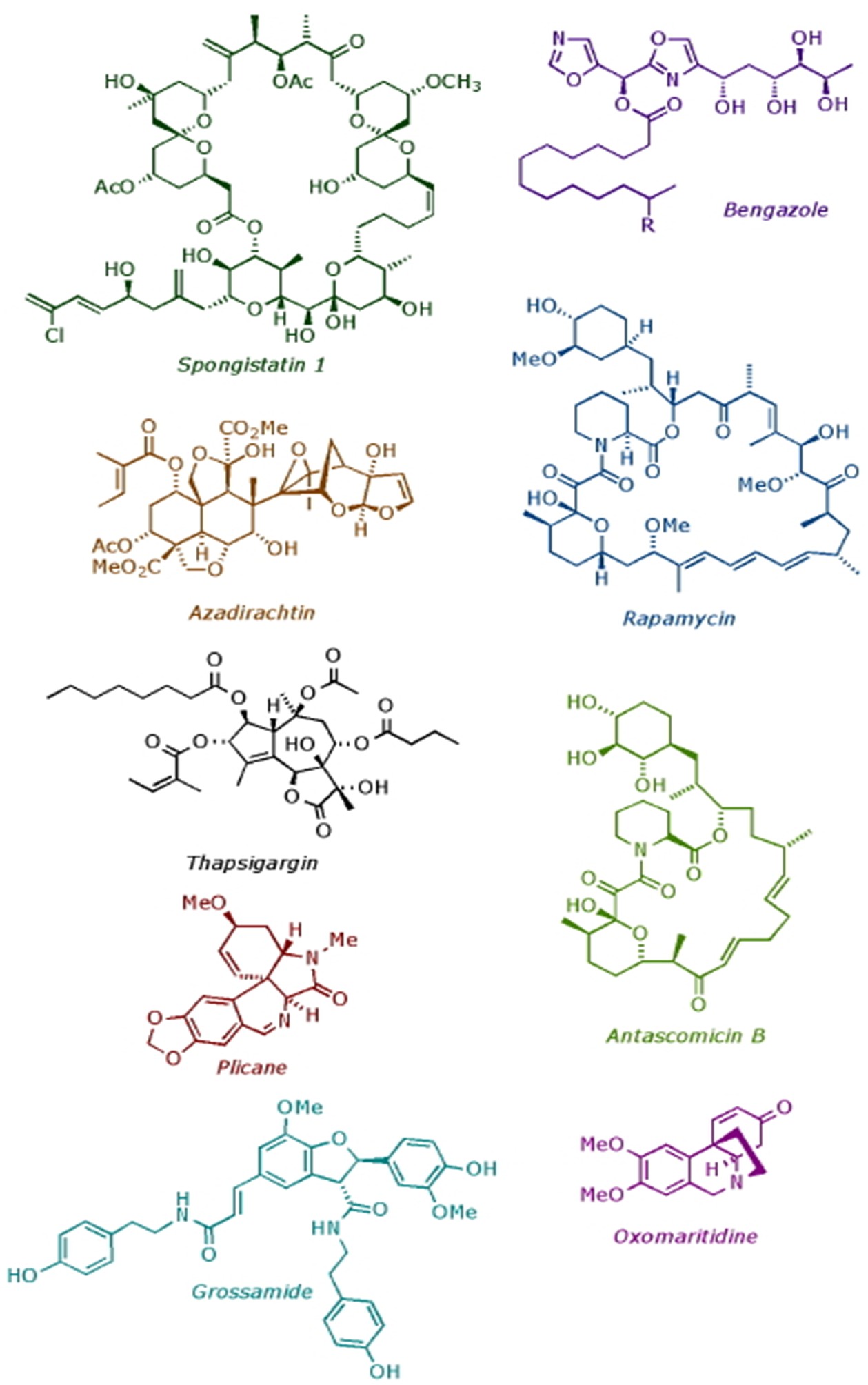
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
A Facile One‐Pot Synthesis of a Trisaccharide Unit from the Common Polysaccharide Antigen of Group B Streptococci Using Cyclohexane‐1, 2‐diacetal (CDA) Protected Rhamnosides
Angewandte Chemie International Edition in English
(2003)
33
2292
(doi: 10.1002/anie.199422921)
CYCLOHEXANE-1,2-DIACETALS IN SYNTHESIS .1. CYCLOHEXANE-1,2-DIACETALS (CDA) - A NEW PROTECTING GROUP FOR VICINAL DIOLS IN CARBOHYDRATES
Angewandte Chemie International Edition
(2003)
33
2290
(doi: 10.1002/anie.199422901)
Microwave assisted Leimgruber–Batcho reaction for the preparation of indoles , azaindoles and pyrroylquinolines
Organic & Biomolecular Chemistry
(2003)
2
160
(doi: 10.1039/b313012f)
A Route to the Thapsigargins from (S)-Carvone Providing a Substrate-Controlled Total Synthesis of Trilobolide, Nortrilobolide, and Thapsivillosin F
Angew Chem Int Ed Engl
(2003)
42
5996
(doi: 10.1002/anie.200353140)
Enantioselective synthesis of the tetrahydrobenzyl-isoquinoline alkaloid (-)-norarmepavine using polymer supported reagents
Heterocycles
(2003)
60
2707
(doi: 10.3987/com-03-9892)
Copper(I)-catalyzed preparation of (E)-3-iodoprop-2-enoic acid: (2-Propenoic acid, 3-iodo-, (2E)-)
Organic Syntheses
(2003)
80
129
The Use of Butane Diacetals of Glycolic Acid as Precursors for the Synthesis of the Phytotoxic Calmodulin Inhibitor Herbarumin II
Helvetica Chimica Acta
(2003)
86
3717
(doi: 10.1002/hlca.200390314)
The synthesis of the anti-malarial natural product polysphorin and analogues using polymer -supported reagents and scavengers
Organic & biomolecular chemistry
(2003)
1
3957
(doi: 10.1039/b308761a)
Preparation of butane-1,2-diacetal-protected l-glyceraldehyde from D-mannitol
Synthesis
(2003)
2004
147
(doi: 10.1055/s-2003-42489)
Synthesis of the C-1−C-28 ABCD Unit of Spongistatin 1
Organic letters
(2003)
5
4819
(doi: 10.1021/ol035849+)
- ‹ previous
- Page 50

