
Director of Research
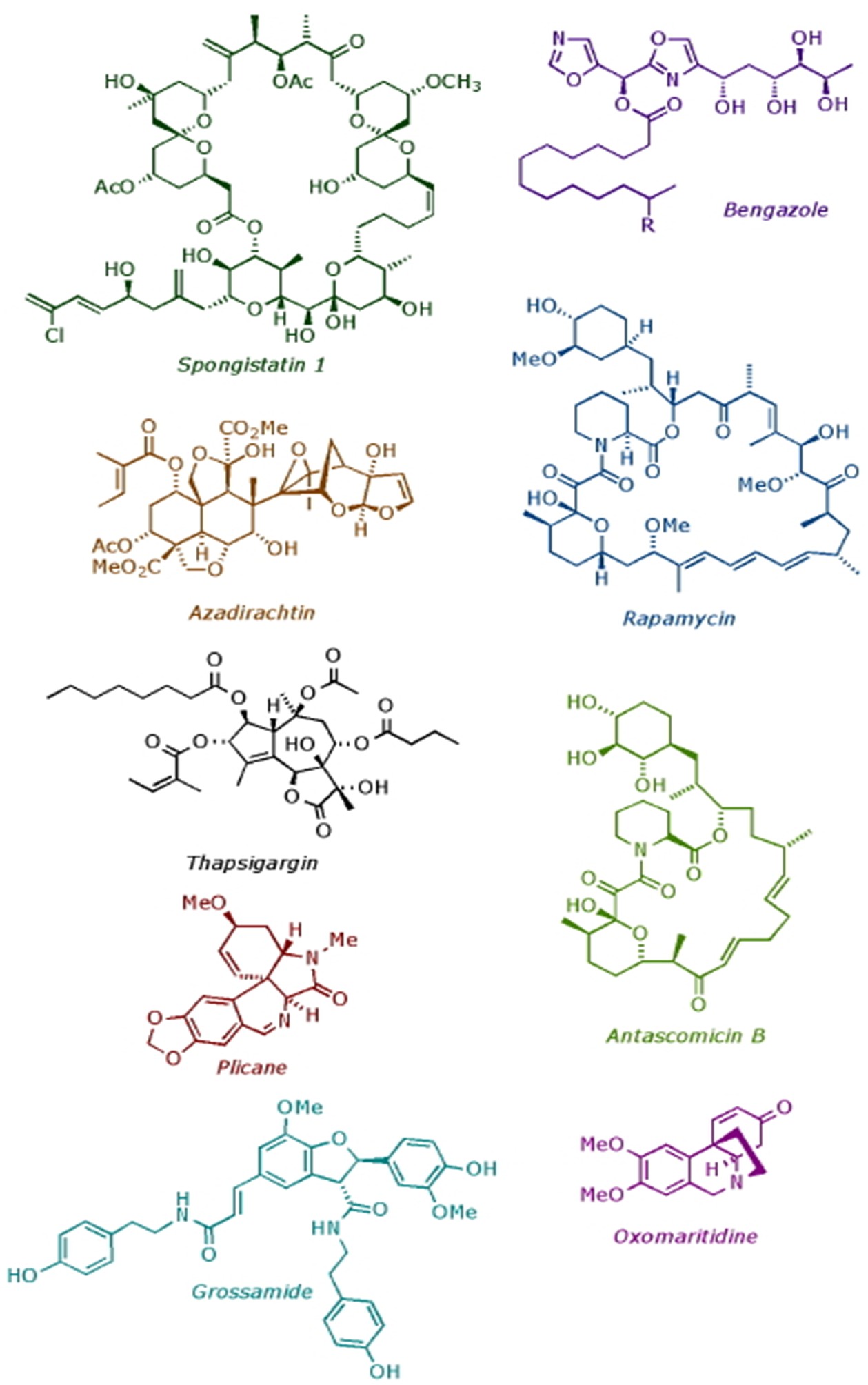
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
An efficient, asymmetric organocatalyst-mediated conjugate addition of nitroalkanes to unsaturated cyclic and acyclic ketones.
Organic & Biomolecular Chemistry
(2006)
4
2039
(doi: 10.1039/b601877g)
A highly enantioselective total synthesis of (+)-goniodiol.
Organic & Biomolecular Chemistry
(2006)
4
1698
(doi: 10.1039/b602805e)
A flow process for the multi-step synthesis of the alkaloid natural product oxomaritidine: A new paradigm for molecular assembly
Chemical Communications
(2006)
2566
(doi: 10.1039/b600382f)
Practical synthesis of (S)-pyrrolidin-2-yl-1H-tetrazole, incorporating efficient protecting group removal by flow-reactor hydrogenolysis
Synlett
(2006)
2006
889
(doi: 10.1055/s-2006.939036)
Diastereoselective aldol reactions with butane-2,3-diacetal protected glyceraldehyde derivatives
Organic & Biomolecular Chemistry
(2006)
4
1471
(doi: 10.1039/b601888b)
Double Conjugate Addition of Dithiols to Propargylic Carbonyl Systems To Generate Protected 1,3-Dicarbonyl Compounds
The Journal of organic chemistry
(2006)
71
2715
(doi: 10.1021/jo052514s)
Preparation of the Neolignan Natural Product Grossamide by a Continuous-Flow Process
Synlett
(2006)
2006
0427
(doi: 10.1055/s-2006-926244)
A New Strategy for Oligosaccharide Assembly Exploiting Cyclohexane‐1,2‐diacetal Methodology: An Efficient Synthesis of a High Mannose Type Nonasaccharide
Chemistry - A European Journal
(2006)
3
431
(doi: 10.1002/chem.19970030315)
Diastereoselective aldol reactions with butane-2,3-diacetal protected glyceraldehyde derivatives (vol 4, pg 1471, 2006)
ORGANIC & BIOMOLECULAR CHEMISTRY
(2006)
4
1612
Asymmetric organocatalytic conjugate addition of malonates to enones using a proline tetrazole catalyst
Chem Commun (Camb)
(2005)
66
(doi: 10.1039/b514636d)
- ‹ previous
- Page 43

