
Developing automated and/or machine-assisted processes for fully utilising substances and reactive intermediates that we cannot easily harness, process and exploit using conventional laboratory techniques is one of our main goals.
Natural Product Targets and Past Achievements Using Flow Synthesis Methods
Spirangien A and B
One of our current flow total synthesis targets are the spirangiens. On completion this synthesis will constitute a significant demonstration of the power of combined flow methods. We have made good progress in this area using specially developed flow chemistry devices and methods. Spirangiens are polyketides consisting of 14 stereocentres, a densely functionalised spiroketal core and an unstable Z–E–Z–E–Zconjugated polyene fragment. Our present synthesis utilises a number of highly stereoselective reactions, including asymmetric hydrogenation, crotylation and alkyne additions. To date, a flow-based synthesis of a key intermediate has been developed which employs polymer-supported reagents, inline analytical devices and our newly developed gas-liquid reactor as part of the flow sequence.
O-Methyl-Siphonazole
The bisoxazole containing natural product O-methyl siphonazole was assembled using a suite of microreactors via a flow-based approach in concert with traditional batch methods. The use of a toolbox of solid-supported scavengers and reagents to aid purification afforded the natural product in a total of nine steps.
Pseudomonas Quinolone Signal
In a collaborative effort with Dr David Spring and Dr Mark Ladlow, a tandem reaction process was devised that allows a two-step synthesis of PQS (and analogues) on a multi-gram scale from readily obtainable or commercially available materials. Access to these materials will help us to understand PQS-mediated quorum sensing systemsand thus may facilitate the development of methods that could modulate these signalling networks in a useful fashion, with possible therapeutic applications in the treatment of human bacterial infections. Below is a schematic of the synthesis of PQS using a Uniqsis FlowSyn™ continuous flow reactor.
Grossamide
In 2006, we reported the first enantioselective total synthesis of 2-aryl-2,3-dihydro-3-benzofurancarboxyamide neolignan, grossamide, using a fully automated and scalable flow reactor. Its preparation using flow chemistry methods involved amide coupling of tyramine and ferulic acid using an immobilized HOBt cartridge followed by an oxidative dimerisation and an intramolecular cyclisation. For the oxidation step, this precursor was diluted (3:1) with a second input solution containing hydrogen peroxide urea complex and sodium dihydrogen phosphate buffer. The combined flow stream was then passed through a pre-packed column with the enzyme horseradish peroxidase (II) supported on silica gel which ultimately gave the desired natural product in excellent overall yield.
Oxomaritidine
This was the first multi-step synthesis of a natural product using flow methods and techniques to be reported in the literature. We did not need to isolate any intermediates along the way nor did we need to perform any distillations, crystallisations or perform any column chromatography throughout the whole synthesis. The time saved by using these flow through methods compared to conventional procedures was dramatic.
Medicinal Compounds in Flow
Enabling tools and technologies such as flow synthesis devices and approaches, solid-supported reagents, scavengers and computer-led processes have been assisting us in our medicinal chemistry programmes. Here we show some of the flow processes and new methods that we have developed for a number of pharmaceutical compounds.
Synthesis of biologically active agents using flow chemistry
Tamoxifen
In this synthesis we used a newly developed, chemically resistant, peristaltic pumping system to pump organometallic reagents (nBuLi), Grignard reagents, and DIBAL-H. We have shown how it can be used in common transformations (metal–halogen exchange, addition, addition–elimination, conjugate addition, and partial reduction) and telescoping of anionic reaction products. This platform allowed truly continuous pumping of these highly reactive substances, over several hours, to generate multigram quantities of products. This work culminated in an approach to the telescoped synthesis of (E/Z)-tamoxifen using continuous-flow organometallic reagent-mediated transformations.
Meclinertant (SR 48692)
We developed an improved synthesis of the neurotensin antagonist biological probe SR 48692. The preparation includes an number of chemical conversions and strategies involving the use of flow chemistry platforms which helped overcome some of the limiting synthetic transformations in the original chemical route .
Fanetizole
We had previously developed a tube-in-tube reactor based on a semipermeable polymer membrane to enable the transfer of gases into liquid flow streams. and here, we demonstrate the scalability and throughput of this reactor when used with ammonia gas. This was made possible by a the inclusion of a titration method to assess parameters including the liquid and gas configuration, reactor temperatures, flow rates, and solvent polarity. These data were then employed in a scaling-up process affording alkyl thioureas which were ultimately used in a telescoped procedure for the preparation of anti-inflammatory agent fanetizole on a multigram scale.
Gleevec (Imatinib)
We reported a concise, flow-based synthesis of Imatinib, a compound used for the treatment of chronic myeloid leukaemia. This synthesis was conducted using tubular flow coils or cartridges packed with reagents or scavengers to bring about all the steps of the preparation of the API. Furthermore, we devised a simple solution to in-line evaporation and solvent switching during a flow chemistry application.
Synthesis of a δ-Opioid Receptor Agonist
This article describes the design, optimisation and development of a continuous flow synthesis of N,N-diethyl-4-(3-fluorophenylpiperidin-4-ylidenemethyl)benzamide, a potent delta-opioid receptor agonist developed by AstraZeneca. With a ReactIR flow cell used as a monitoring device, initiation of the fourth input flow stream was precisely controlled for the dehydration step using the Burgess reagent.
Casein Kinase I Inhibitors
Overall a collection of twenty diverse analogues of a casein kinase I inhibitor has been synthesised by changing the three principle chemical inputs.
5HTIB Antagonist
This article describes the continuous flow synthesis of 6-methoxy-8-(4-methyl-1,4-diazepan-1-yl)-N-(4-morpholinophen-yl)-4-oxo-1,4-dihydroquinoline-2-carboxamide, a potent 5HT1B antagonist developed by AstraZeneca. Notably, in this work is the use of Hastelloy flow coils to bring about high temperature reactions commonly achieved using microwave methods.
Publications
Tamoxifen: Continuous flow-processing of organometallic reagents using an advanced peristaltic pumping system and the telescoped flow synthesis of (E/Z)-tamoxifen P.R.D. Murray, D.L. Browne, J.C. Pastre, C. Butters, D. Guthrie, S.V. Ley, Org. Proc. Res. Dev. 2013, 17, 1192-1208.
Meclinertant (SR 48692): A machine-assisted flow synthesis of SR48692: a probe for the investigation of neurotensin receptor-1 C. Battilocchio, B.J. Deadman, N. Nikbin, M.O. Kitching, I.R. Baxendale, S.V. Ley, Chem. Eur. J. 2013, 19, 7917-7930.
Fanetizole: Scaling-up of continuous flow processes with gases using a tube-in-tube reactor: in-line titrations and fanetizole synthesis with ammonia J. Pastre, D.L. Browne, M. O’Brien and S.V. Ley, Org. Proc. Res. Dev. 2013, 17, 1183-1191.
Gleevec: An automated flow-based synthesis of imatinib: the API of gleevec M.D. Hopkin, I.R. Baxendale, S.V. Ley, Chem. Commun. 2010, 46, 2450-2452
Opioid Receptor Antagonist: A continuous flow process using a sequence of microreactors with in-line IR analysis of the preparation of N, N-diethyl-4-(3-fluorophenylpiperidin-4-ylidenemethyl) benzamide as a potent and highly selective gamma-opioid receptor agonist Z. Qian, I.R. Baxendale, S.V. Ley, Chem. Eur. J. 2010, 16, 12342-12348
Casein Kinase Inhibitors: Application of flow chemistry microreactors in the preparation of casein kinase I inhibitors
F. Venturoni, N. Nikbin, S.V. Ley, I.R. Baxendale, Org. Biomol. Chem. 2010, 8, 1798-1806
5HT1B Antagonist: A flow process using microreactors for the preparation of a quinolone derivative as a potent 5HTIB antagonist Z. Qian, I. R. Baxendale, S.V. Ley, Synlett, 2010, 505-508
Flow Generated Sequential Complexity
Biomimetic syntheses of alkaloids
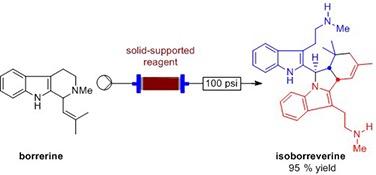
Borrerine-derived alkaloids such asor the flinderoles have shown significant antimalarial activity including in chloroquine-resistant strains of Plasmodium falciparum [1-6].
Using flow chemistry platforms, we have begun investigating the syntheses of these bisindol compounds and our initial results have shown that by passing a solution of borrerine through a cartridge containing a solid-supported Lewis acid we can obtain isoborreverine selectively without any purification.
References
Heterocylces
In our 2011 Molecular Diversity review article [1] we described an overview of recent research studies towards the preparation of heterocyclic compounds especially those of medicinal interest.
Triazoles
We devised a general protocol for the in-line preparation and purification of aryl azide intermediates from which a series of 5-amino-4-cyano-1,2,3-triazoles from anilines were prepared in a fully automated fashion. In this work we also evaluated the use of an infrared flow device (the ReactIR 45m) as a tool for real-time monitoring of potentially hazardous intermediates [2].
In another route to triazoles, we have also used the Seyferth-Gilbert reagent 1 in a flow system to synthesise terminal alkynes and employed these in the preparation of triazoles such as 3 from alcohol 2in a three-step oxidation/homologation/copper(I)-catalyzed azide-alkyne cycloaddition sequence without isolation of intermediates [3].
Thiazoles and Imidazoles
A scalable method for the preparation of 4,5-disubstituted thiazoles and imidazoles using a modular flow microreactor has been devised [4]. The process makes use of microfluidic reaction chips and packed immobilized reagent columns to effect bifurcation of the reaction pathway to afford the different products in a selective fashion.
Pyrrolidines
We have synthesised a series of trisubstituted drug-like pyrrolidines [5] via an efficient use of microreactors to bring about useful cycloaddition processes.
Nitropyrrolidines and nitropyrroles
We have further reported on the dipolar cycloaddition reactions with unstabilized azomethine ylids and nitro alkenes to generate 3-nitropyrrolidines using flow chemistry methods [6]. In later work we describe the development of a three-component coupling reaction between glycine esters, aldehydes and nitro alkenes. In order to demonstrate the utility of flow technology in concert with heterogeneous reagents and scavengers for complex reaction sequences an in-line oxidation resulting in the conversion of tetra-substituted pyrrolidines to their pyrrole congeners was also developed [7].
In other work we have devised novel pyrrole syntheses using flow reactors by combining tosyl isocyanide and ethyl chloroformate with nitrostyrenes to afford nitro-substituted pyrroles in a single step. Catch-and-release protocols were used to purify the products following their synthesis [8].
Yne-ones
We have also reported on the palladium-catalysed acylation of terminal alkynes for the preparation of yne-ones which, after in-line reagent stream-splitting, gave various heterocycles [9].
Oxazoles
A multipurpose mesofluidic flow reactor capable of producing multi-gram quantities of material has been developed as an automated synthesis platform for the rapid on-demand synthesis of key building blocks and small exploratory libraries [10]. The reactor was configured to provide the maximum flexibility for screening of reaction parameters that incorporated on-chip mixing and columns of solid supported reagents to expedite the chemical syntheses.
Quinoxalines
A flow method for the synthesis of aliphatic and aromatic diazoketones from acyl chloride precursors has been developed and used to prepare quinoxalines in a multistep sequence without isolation of the potentially explosive diazoketone building blocks. The protocol showcases an efficient in-line purification using supported scavengers with time-saving and safety benefits and in particular a reduction in the operator’s exposure to carcinogenic phenylenediamines [11].
Imidazopyridazines
In this article we demonstrate how a combination of enabling technologies such as flow synthesis, solid-supported reagents and scavenging resins utilised under fully automated software control can assist in typical medicinal chemistry programmes. In particular automated continuous flow methods have greatly assisted in the optimisation of reaction conditions and facilitated scale up operations involving hazardous chemical materials. Overall a collection of twenty diverse analogues of a casein kinase I inhibitor has been synthesised by changing three principle chemical inputs [12].
Butane-2,3-diacetals
The continuous flow synthesis of butane-2,3-diacetal protected derivatives has been achieved using commercially available flow chemistry microreactors in concert with solid supported reagents and scavengers to provide in-line purification systems [13]. The BDA protected products are all obtained in superior yield to the corresponding batch processes and can then be used as important starting materials for various natural product synthesis programmes.
Quinolones
The quinolone derivative shown below is a potent 5HT1B antagonist developed by AstraZeneca. The continuous flow synthesis (the final steps shown below) of this pharmaceutical agent was completed using a combination of flow microreactors, while incorporating polymer-supported reagents and scavengers to aid reaction telescoping and purification [14]. The result is encouraging, as it clearly demonstrates that multi-step sequences can be incorporated into flow chemistry platforms leading to polyfunctional molecules of biological interest. Moreover, we were able to improve on the overall yield via a batch method using the new reactors.
Publications
1. The flow synthesis of heterocycles for natural products and medicinal chemistry applications
M. Baumann, I.R. Baxendale, S.V. Ley
Mol. Div. 2011, 15, 613-630
2. Fully automated, multistep flow synthesis of 5-amino-4-cyano-1,2,3-triazoles
C.J. Smith, I.R. Baxendale, H. Lange, S.V. Ley
Org. Biomol. Chem. 2011, 9, 1938-1947
3. MultiStep synthesis using modular flow reactors: Bestmann-Ohira reagent for the formation of alkynes and triazoles
I.R. Baxendale, S.V. Ley, A.C. Mansfield, C.D. Smith
Angew. Chem. Int. Ed. 2009, 48, 4017-4021
4. A bifurcated pathway to thiazoles and imidazoles using a modular flow microreactor
I.R. Baxendale, S.V. Ley, C.D. Smith, L. Tamborini, A.F. Voica
J. Comb. Chem. 2008, 10, 851-857
5. Synthesis of a drug-like focused library of trisubstituted pyrrolidines using integrated flow chemistry and batch methods
M. Baumann, R.E. Martin, C. Kuratli, J. Schneider, I.R. Baxendale, S.V. Ley
A.C.S. Comb. Sci. 2011, 13, 405-413
6. Synthesis of nitropyrrolidines via dipolar cycloaddition reactions using a modular flow reactor
M. Baumann, I.R. Baxendale, S.V. Ley
Synlett 2010, 749-752
7. Synthesis of highly substituted 3-nitropyrrolidines and 3-nitropyrroles by a multicomponent multi-step flow sequence
M. Baumann, I.R. Baxendale, J. Wegner, A. Kirschning, S.V. Ley
Heterocycles 2010, 82, 1297-1316
8. A base-catalysed, one pot, three component coupling reaction leading to nitrosubstituted pyrroles
I.R. Baxendale, C.D. Buckle, S.V. Ley, L. Tamborini
Synthesis 2009, 9, 1485-1493
9. Multi-step synthesis using modular flow reactors: the preparation of yne-ones and their use in heterocycle synthesis
I.R. Baxendale, S.C. Schou, J. Sedelmeier, S.V. Ley
Chem. Eur. J. 2010, 16, 89-94
10. A fully automated continuous flow synthesis of 4,5-disbustituted oxaxoles
M. Baumann, I.R. Baxendale, S.V. Ley, C.D. Smith, G.K. Tranmer
Org. Lett. 2006, 8, 5231-5234
11. Safe and reliable synthesis of diazoketones and quinoxalines in a continuous flow reactor
L. J. Martin, A.L. Marzinzik, S.V. Ley, I.R. Baxendale
Org. Lett. 2011, 13, 320-323
12. Application of flow chemistry microreactors in the preparation of casein kinase I inhibitors
F. Venturoni, N. Nikbin, S.V. Ley and I.R. Baxendale
Org. Biomol. Chem. 2010, 8, 1798-1806
13. The continuous flow synthesis of butane 2,3-diacetal protected building blocks using microreactors
C.F. Carter, I.R. Baxendale, J.B.J. Pavey, S.V. Ley
Org. Biomol. Chem. 2010, 8, 1588-1595
14. A flow process using microreactors for the preparation of a quinolone derivative as a potent 5HTIB antagonist
Z. Qian, I. R. Baxendale, S.V. Ley
Synlett 2010, 505-508

